Which of the Following Best Describes a Pi Bond.
Lateral overlapping of orbitals will form pi bonds. Hence it will always form a sigma bond.

Wave Function Wave Function Chemistry Physics
Which of the following best describes the type of bonding in a sample of CO2 s answer choices.

. 5Describe a cycloaddition reaction. Strong single covalent bonds with weak intermolecular forces. Describe a pi bond.
Explore more such questions and answers at BYJUS. Overlap of two f orbitals. A side by side overlap of p orbitals B end to end overlap of p orbitals C s orbital overlapping with the end of a d orbital D overlap of two d.
AIf TE describes the reactionthen the outcome is given by AC. Out of phase combination of these two p orbitals give π MO. A 0 b 1 c 2 d 3 e 4 23.
They are formed by the axial overlap of orbitals. The strong electrostatic attraction between cations and anions causes the formation. Atomospheric methane is a potent greenhouse gas.
Sigma pi A mixture of equal amounts of two enantiomers. By 2008 however global methane levels which had stayed mostly flat since 1998 had risen to 1800 nmolmol. Which of the following statements best describes the relative bond dissociation energies of the sigma and pi bonds present in the carbon-carbon double bond of an alkene.
- Pi molecular orbitals are used to form pi bonds which are involved in double bonds and triple bonds. So as given in the diagram the opposite charges are overlapping each other hence it is a pi antibonding molecular orbital. They are formed by the sideways overlap of parallel orbitals.
A lattice of positive and negative ions held together by electrostatic forces. One of these orbitals forms a pi bond with a 2p orbital of the oxygen atom on the left and the other forms a pi bond with a 2p orbital of the oxygen. The atomic orbitals and molecular orbitals both show the probability of the finding electron in a molecule.
Which of the following best describes the formation of pi bonds. The pi bonds are weaker bonds than the sigma bonds. It is denoted by.
Hence option D is the correct answer. BIf PO describes the reactionthen the outcome is given by AC. They are formed by the sideways overlap of parallel orbitals.
CIf TO describes the reactionthen the outcome is given by AC. Which of the following statements best describes the relative bond dissociation energies of the sigma and pi bonds present in the carbon-carbon double bond of an alkene. The CN bonds in urea are shorter than might be expected for a.
A sigma pi B pi sigma C sigma pi D cannot be estimated. The concentration of methane in Earths atmosphere in 1998 expressed as a mole fraction was 1745 nmolmol ppb. Side by side overlap of p orbitals.
A end to end d orbital overlap b s to s orbital overlap c side by side p orbital overlap d side by side sp3 orbital overlap 22. End to end overlap of p orbitals. Dboth A and B.
Play this game to review Chemical Bonds. π -bonds are weak bonds that are formed by side-wise overlap of parallel p-orbitals. Each molecular orbital has two electrons with the opposite spin.
How many pi bonds are present in a molecule of NH 3. A Strong covalent bonds between atoms with similar electronegativities. The electrons which take part in the formation of pi covalent bonds are called pi electrons.
We find pi bonds in alkenes and alkynes. Describe a pi bond. Three pi bonds two sigma bonds and two pi bonds one sigma bond and one pi bond two sigma bonds and one pi bond one sigma bond and two pi bonds.
2 Which of the following bonds would be best categorized as covalent. QUESTION 32 Which of the following describes a triple bond. N-F A I only B II only C III only D I and III E I II and III 3 Which of the following BEST describes the bonding found within solid Al 2 O 3.
Which of the following best describes a pi bond. Which of the following best describes the orbitals involved in the formation of C O bond in acetone shown below sigma C_sp2 - O_sp2 and pi C_sp2 - O_sp2 sigma C_sp2 - O_sp2 and pi C_p - O_p sigma C_sp3 - O_sp2 and pi C_p - O_p sigma C_p - O_p and pi C_sp2 - O_sp2 sigma C_sp - O_sp and pi C_p - O_p. AThe reactant has lost a sigma bondthe product has a new sigma bondand the pi.
S orbital overlapping with the end of a d orbital. Which of the following orbital overlapping will form Pi bond. Hence the correct option is D.
The covalent bond which is formed by lateral overlapping of the half-filled atomic orbitals p - orbitals of atoms is called a pi bond. S orbital will always overlap axially with any other orbital due to its symmetric structure. - For example the carbon atom in carbon dioxide CO 2 has two perpendicular 2p orbitals.
Correct option is D An antibonding π orbital best describes the diagram of a molecular orbital. The Correct Answer is C Solution. They are formed by the axial overlap of either s or p orbitals.
P orbital overlapping with a d orbital. Describe how sigma σ and pi pi bonds are formed. Asked Aug 6 2019 in Chemistry by lpngal.
They are formed by the sideways overlap of an s and p orbital. In case of a single bond it contains only one sigma bond in case of double bond it contains one sigma and one pi bond and in case of triple bond it contains one sigma and two pi bonds. Due to the side-wise overlap which takes place at a distance the bond is weaker than the σ -bond.
See the answer See the answer See the answer done loading. Strong multiple covalent bonds including pi bonds with weak intermolecular forces. This problem has been solved.
Enone of the above. The carbon-carbon double bond in ethene consists of one sigma bond formed by the overlap of two sp 2 orbitals and a second bond called a pi bond which is formed by the side-by-side overlap of the two unhybridized 2p z orbitals from each carbon. Two p orbitals laterally overlap to form pi bond.
Total 1 mark 85. Which of the following best describes the type of bonding in. Hence option C is the correct answer.

Chemsolve Net Nanotechnology Chemistry Classroom Chemistry Lessons
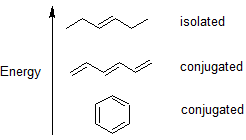
2 4 2 4 Conjugated Pi Bond Systems Chemistry Libretexts

Practical Research 2 Linabuan National High School Senior High Ma Nelyn Amor I Ricarto T I Content National High School Research Quantitative Research

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Molecular Geometry Chemistry

Sigma And Pi Bonds Definition Examples Diagrams

Zodiac Ifunny Horoscope Pisces Pisces Quotes Zodiac Mind

Pi Bond Definition Explanation Examples With Illustrations

Pin By Ginn On Zodiac Astrology N Interesting Wallpaper Horoscope Pisces Pisces Quotes Zodiac Signs Pisces

Pineapple Nomenclature Book Pineapple Pineapple Flowers Montessori Lessons

New Standalone Cr10 Cr10s Tornado En Etude By Razorbac Thingiverse 3d Printer Machine 3d Printer 3d Printing Diy

Whatsapp Is Updating Its Privacy Rules Here S What You Need To Know In 2021 Business Communication Social Media Need To Know

Sorority Paddles From Momma Back Sorority Paddles Sorority Crafts
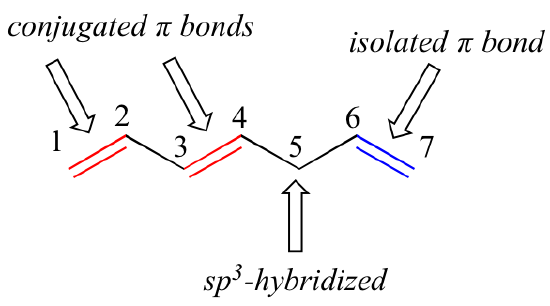
2 4 2 4 Conjugated Pi Bond Systems Chemistry Libretexts

Pi Bond Definition Explanation Examples With Illustrations
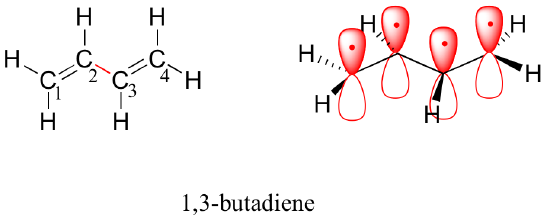
2 4 2 4 Conjugated Pi Bond Systems Chemistry Libretexts

Molecular Orbital Diagram Wikipedia The Free Encyclopedia Diagram Molecular Science Chemistry



Comments
Post a Comment